The Novavax Covid-in-2015 mishap
Neither regulators nor sponsors seem to really be reading these files
**Für deutsche Leser empfehle ich das DeepL-Plugin, sonst ist hier der leider minderwertige Google-Übersetzer-Link: https://modarnlife-substack-com.translate.goog/p/the-novavax-covid-in-2015-mishap?_x_tr_sl=auto&_x_tr_tl=de&_x_tr_hl=en-US&_x_tr_pto=wapp
The initial Novavax marketing authorisation documents published by EMA CDP contain m5353-iss.pdf, an “Integrated Summary of Safety of Other Novavax Recombinant Nanoparticle Vaccine Antigens with Matrix-M1TM Adjuvant” dated April 14th 2021.
Page 9 explains the purpose of this document as follows:
Clinical trials supporting the SARS-CoV-2 rS with Matrix-M1 adjuvant clinical development program are summarized in Table 1. Available data from each of these trials will be provided in individual interim reports; no integrated summary of safety data from the SARS-CoV-2 rS with Matrix-M1 adjuvant studies is available at this time given the urgent need to rapidly prepare data for regulatory submissions during the ongoing global coronavirus pandemic.
To supplement the lack of available long-term safety data (≥ 6 months) in the ongoing clinical trials of SARS-CoV-2 rS with Matrix-M1 adjuvant (Table 1), an integrated analysis of safety was performed in 2,574 adult participants 18 years of age and older across 5 Novavax-sponsored clinical trials of other recombinant nanoparticle vaccine antigens using the same manufacturing platform technology as SARS-CoV-2 rS administered with the same Matrix-M1 adjuvant with safety follow-up ranging from 6 months to 1 year (Table 2).
In other words, they couldn’t (be bothered to) summarize the huge amount of patients they’d enrolled in the Covid trials and instead offered up a fraction of the sample size from five previous trials. The mentioned Covid trials are also included in the submission, but not in summarized form.
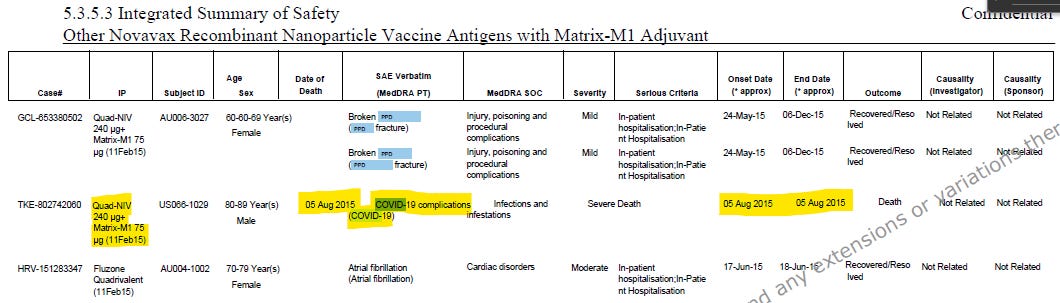
Two of the safety narratives included in m5353-iss.pdf are allegedly Covid deaths from 2015. One even claims it was listed on the death certificate. There are also four 2015 and one 2016 Covid case listed in tables, but as they did not result in death they didn’t get a narrative.
All anachronistic Covid mentions in the safety summary are listed below. The table entries are found on page 115, 119, 120, 121, 122 and 124.


It quickly becomes clear that the main error is all years were replaced by 2015. The 42-page SAE line listing appendix starting on page 93 contains approximately 300-320 narratives, and all have dates in 2015, except for ~25 entries with end/start+end dates in 2016. The table is even titled “01-JAN-2014 To 17-MAR-2021”.
Novavax shit the bed badly assembling this document and EMA perhaps even more so releasing it in this state. All these Covid mentions are associated with just one of the summarized studies, trial qNIV-E-301, which ran from 14. October 2019 to 29 October 2020. In other words, the unpaid intern running on stale coffee and merchandising sweet bags hotglueing this document together 46 minutes before upload to EMA managed to replace all the years in the m5353-iss.pdf with 2015. The Covid cases did not occur in 2015, as the document claims, but in 2020. The trial site linked above has results posted, and lo and behold, there are 7 Covid diagnoses listed caught in the 1-year followup. Perhaps dutiful trial participants notifying Novavax?
Of approximately 320 entries in the appendix table, 25-30 have end/start+end dates in 2016. The studies they are taken from have the following dates:
EBOV-H-101 11.02.2015 - 19.04.2016
RSV-E-205 16.01.2017 - 24.03.2018
tNIV-E-101 18.09.2017 - 26.10.2018
qNIV-E-201 24.09.2018 - 16.04.2019
qNIV-E-301 14.10.2019 - 29.10.2020
Perhaps they had the dosing intervals stored and populated the narratives with corresponding data from the studies, but it got stuck on the Ebola trial, something like that? There is also the distant possibility this is some form of data anonymisation, yet this is not really plausible due to there being redactions. As for the smattering of 2016 dates, perhaps these were “year n+1” entries, or a confused last minute edit? It might well be just a colossal mistake that was just noticed at the last minute, unlikely as that sounds. As the cases are legitimate, the error is also ultimately meaningless; what’s actually outrageous is the provided sample size.
The real scandal here is the inexcusable cherrypicking EMA let Novavax get away with in this document: the total sample size of summarized patients in m5353-iss.pdf is 2,547 who received a Novavax vaccine, 1,582 receiving a comparator flu jab, and 73 receiving placebo, adding up to 4,202.
Here is the list of 2013-2020 Novavax clinical trials and their enrollments.
The highlighted studies are the ones included in the summary. The SAE appendix table opens with 01.01.2014, so subjects from all the trials above could have theoretically been eligible, and their enrollment adds up to 21,507 (excluding the highlighted ones).
Novavax could have supplied an integrated summary of safety with n=25k, yet they chose to only summarize data from less than a fifth of their trial subjects in the timeframe. They even avoided adding the safety data of an 11k participant Phase 3 RSV trial. And regulators were apparently just fine with that, not that they seem to have read the document closely enough to notice false dates.
Reading the document and roughly summarizing the safety data, which Novavax abstained from doing “given the uniqueness of 4 of the vaccine antigens (EBOV GP, RSV F, Tri-NIV, and Quad-NIV) and dosing regimens (one versus two doses).” (p61), only really tells you they test their vaccines on old people (mean age 68,55 years) and only shows safety data for 265 two-dose subjects (p54). Yes, only ten percent of summarized subjects had received two doses of Matrix-M adjuvanted vaccines.



Then they have the nerve to say this (p61 again):
In general, frequencies of solicited local and systemic TEAEs were increased in recipients who received Matrix-M1-adjuvanted vaccines (compared to those who received vaccines without Matrix-M1 adjuvant) and in recipients who received two-dose Matrix-M1-adjuvanted vaccine (compared to those who received one-dose Matrix-M1-adjuvanted vaccine).
For my (quite long) twitter thread of notes on the documents, see here and then here. Please excuse some rather embarrassing arithmetic mistakes, I will be sorting that out in due course.
For everything I’ve rehosted from the EMA Clinical Data Publication portal, including the original Novavax documents, see this article:
EMA Clinical Data Publication rehosting
Long story short: as a EU/EEA citizen, you can access a pretty large EMA repository of clinical study reports. They’re quite a bit more redacted than the PHMPT files, but there is some form of documentation for every authorization specifically of the Covid products.











an absolute shit show.
a dump to confuse and distract researchers like you?
The FDA and CDC are actively pursuing malfeasance. The EMA is behind, but catching up.