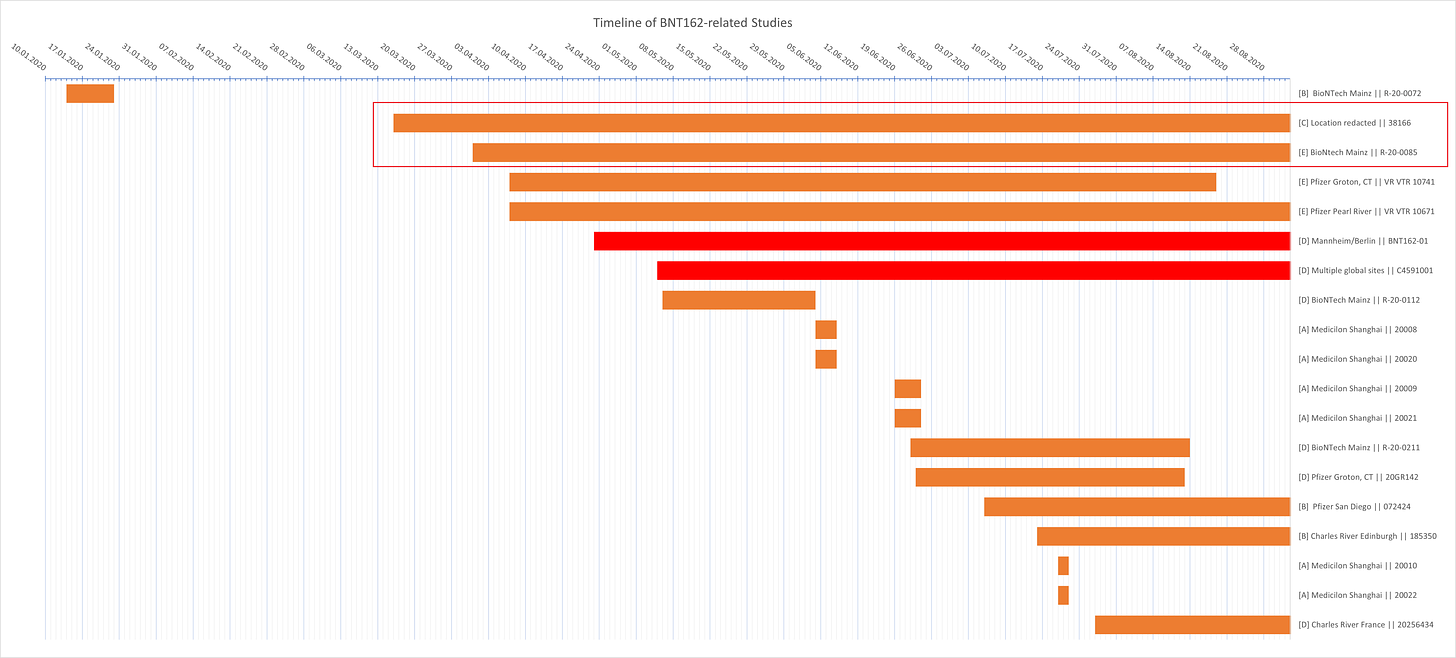
In a recent twitter conversation with
concerning his excellent breakdown of the formidably immense Study 38166, I realized that the switch from BNT162b2v8 to BNT162b2v9 can be quite precisely triangulated from available data beyond what I have explored so far (English translation link can be found at the beginning of the article).You might also want to read the section concerning “batches from the future” in Study 38166 for additional clarity.
I will be looking at the following two studies and their Certificates of Analysis:
Study 38166 - Repeat-dose toxicity
Candidates: BNT162a1 - b1 - b2v8 - c1
Study R-20-0085 - In vivo immunogenicity
Candidate: BNT162b2v9
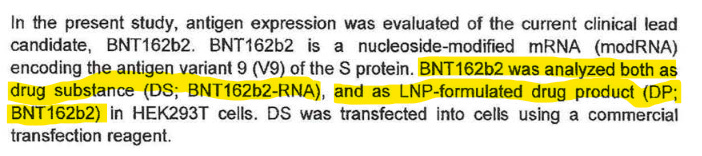
BioNTech made the RNA (drug substance, DS) and Polymun loaded it into LNPs, thus creating the drug product (DP).
The data points we have are the following:
March 16 - Study 38166 begins
March 19 - BioNTech DS release of first BNT162b2v9 batch (Study R-20-0085)
March 26 - Polymun release of all DP charges used in Study 38166
March 31 - Study R-20-0085 begins
April 9 - Polymun DP release of the first BNT162b2v9 batch
BioNTech DS release dates for BNT162a1, b1, and c2 can be found in Study R-20-0112, yet to the best of my knowledge there is currently no BioNTech DS release page for BNT162b2v8. Study 38166 only has the Polymun DP release pages.
March 5 - BNT162b1 BioNTech DS release
March 6 - BNT162a1 BioNTech DS release
March 9 - BNT162c2 BioNTech DS release
It stands to reason that BNT162b2v8 DS was released by BioNTech at some point after March 5 and at least a few days before March 19, when the first b2v9 DS was released. With all of that out of the way, let’s take a closer look what this actually entails.
The first drug product batch of b2v8 was released by Polymun on March 26 to be used in Study 38166 (Study 38166 perplexingly claims to have received b2v8 DP on March 20).
R-20-0085, the next non-clinical study, begins on March 31 and concerns only b2v9 (never mind that b2v9 DP release was April 9.. awkward).
So BioNTech received the first b2v8 DP on March 26 or 27 (accounting for transport between Austria and Germany), yet had already finished DS production of the successor b2v9 on March 19.
There are still a minimum of two unavailable studies concerning b2v8, R-20-0054 and R-20-0360. In order for these studies to have informed the decision to switch from v8 to v9, they will have to have occurred before March 12-13, due to the approximate DS production time of one week (b2v9 DS release on March 19). Furthermore, in order to fit timewise, these studies must have used pure DS (just modRNA) instead of finished DP (modRNA-LNP), as was the case later on in study R-20-0211.
This still leaves the margin incredibly, if not impossibly, close: b2v8 DS release can be assumed to have occurred somewhere between March 5-9 and begin of b2v9 DS production was (March 19 minus ~1 week) somewhere around March 12.
That leaves between 3 and 7 days to test b2v8 and decide to change b2v8 codon optimization.
Ignoring the question of safety, is this even technically plausible?
Addendum: the difference between BNT162b2v8 and v9 seems to be “additional cytosine ribonucleotides”.





I know why the location of rat study 38166 was redacted. It took place at the Laboratory of Pharmacology and Toxicology in Hamburg in March 2020 - but they were being investigated for poor animal welfare and their license was suspended - date was later changed to September 2020.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9965811/
https://www.europarl.europa.eu/doceo/document/E-9-2020-005148_EN.html
wouldn't have been GLC compliant
Thanks very much for this. This Rat study was continued on Humans.
The German trial had two parts, using 512 volunteers, (some of whom were paid) in 5 locations, Part A and Part B. Due to changes in the overall clinical development plan, Part B was abandoned.
It used the four different experimental jabs: BNT162a1, BNT162b1, BNT162b2, and BNT162c2
https://geoffpain.substack.com/p/pfizer-biontech-covid19-jab-multiple