"Comirnaty" is not the "Pfizer-BioNTech Covid-19 Vaccine"
Whatever is being used in the United States, it's not the licensed product...
Update 23.4.2023: repaired the Excel file link to the most up-to-date iteration, I had mistakenly replaced the file with one of the first versions. NZ authorizations included as a separate table. Updated “Other countries” section at the end with (24.4.: yet more) information on South Africa.
Edit 03.01.2024: The statement “The two formulations of the Pfizer-BioNTech COVID-19 Vaccine and COMIRNATY (COVID-19 Vaccine, mRNA) differ with respect to certain inactive ingredients only and have been shown to be analytically comparable.” has been removed from the corresponding document. Here is the April 2023 version which includes the sentence. No further updates to this article (yet).
..and they’re quite explicit in saying so, if you know where to look.
I had previously heard of (and certainly registered) the fact that the Pfizer-BioNTech vaccine was being distributed in the USA with a label saying “Pfizer-BioNTech Covid-19 Vaccine” and stating “For use under Emergency Use Authorization”, not “Comirnaty”.
Pictured below are example vials of American “Pfizer-BioNTech Covid-19 Vaccine” and “Comirnaty”. The latter was definitely in use at all time points in Germany, and I’m fairly certain German labelling has never referenced EUA (or CMA, the Euro counterpart, for that matter). I’ve seen vials throughout the pandemic in Germany starting March 2021 and they were all labeled Comirnaty, but I’m not 100% sure we didn’t also have EUA labelled vials, but I also haven’t found any information suggesting we ever had EUA labelling. Addendum: See end of article for use of EUA-labelled vaccine in Germany. (6.5.2023: added additional proof of EUA-label use in Germany).

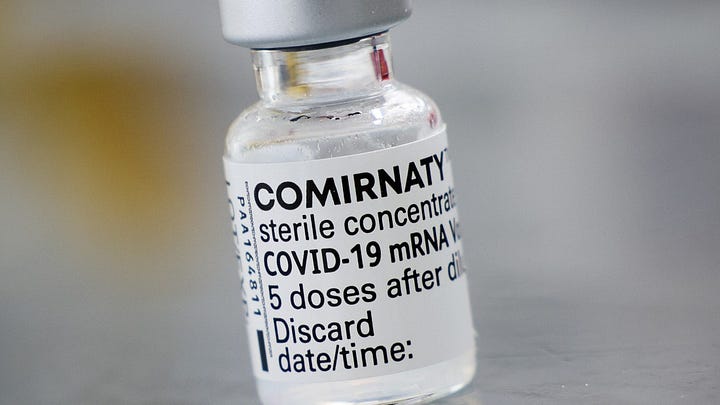
That being said, I am not sure of labelling in other countries. If you have seen vials and/or cartons of the Pfizer/BioNTech vaccine and its labelling in a country other than Germany or the US, please feel free to tell me what the labelling said. Here or on twitter is fine.
Some time after Kevin McKernan published his groundbreaking deep sequencing of the bivalent Pfizer-BioNTech vaccine1 (which is also where I lifted the first picture from), it hit me like a ton of bricks that these vials were labelled differently from what I had personally seen being used in Germany. You know how it is with some things, you register them on a cognitive level but you don’t really grasp the implications until you see it yourself.
@ponykillr on twitter is the author of an impeccably researched substack I’ve reread several times and still not entirely grasped.
More recently, I became aware that Meryl Nass and RFK Jr. have been covering this subject from the beginning.2 34 They have also unsuccessfully sued. If you're aware of more articles on this topic, please feel free to tell me and I'll add them in. Honourable mention: there were in fact nine lots of Comirnaty distributed in the US.
The essence of it is that Comirnaty was never available in the Unites States; instead, there have been regularly updated Emergency Use Authorizations for something called “Pfizer/BioNTech Covid-19 Vaccine”. Even if it were available, Comirnaty has since been EUA’d too.
There are references to the two different formulations littered throughout the documentation. I’ll list what I’ve found in no particular order.
A recent discovery has been the document I should have opened first, the EUA Letter Of Approval for the Pfizer-BioNTech Covid-19 Vaccine.
This lists the individual EUA reissues/revisions and the reasons for them. Every time the scope of use is changed or extended to a new demographic, the EUA is reissued. Notably, when the actual BLA license was issued and expanded in scope, the EUA was concurrently reissued.
Remarkably the bivalent Comirnaty, which has been in use in Europe labelled as such, has not yet received a BLA license from the FDA. On the other hand, this was the case for the monovalent up until August 2021, so perhaps not that remarkable.
It also contains this crucial sentence:
The two formulations of the Pfizer-BioNTech COVID-19 Vaccine and COMIRNATY (COVID-19 Vaccine, mRNA) differ with respect to certain inactive ingredients only and have been shown to be analytically comparable. **This document no longer contains this wording, see 03.01.2024 edit in the beginning of the article.**
Then there are references to be found in Pfizer’s labyrinthine SEC filings, for example their 10-K, the annual report. In the Defined Terms at the beginning, the Comirnaty* entry reads as follows:
Unless otherwise noted, refers to, as applicable, and as authorized or approved, the Pfizer-BioNTech COVID-19 Vaccine, the Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), the Comirnaty Original/Omicron BA.1 Vaccine, and Comirnaty Original/Omicron BA.4/BA.5 Vaccine
It is important for reading the rest of the document that neither Comirnaty Original/Omicron versions are authorized or licensed by the FDA, and it appears the BA1 was never EUA’d in the US, leaving only Pfizer-BioNTech Covid-19 Vaccine (+ Bivalent). On a side note, this is the other way around in Switzerland; there, only the BA1 bivalent has been put on the market, the BA4/BA5 is still under consideration. For additional clarity; tozinameran = wild type, riltozinameran = BA1, famtozinameran = BA4/BA5.
The asterisk leads to following statement:
“Paxlovid and emergency uses of the Pfizer-BioNTech COVID-19 Vaccine or the Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), have not been approved or licensed by the FDA.[…]”
“[..]Emergency uses of the Pfizer-BioNTech COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent have been authorized by the FDA under an EUA to prevent COVID-19 in individuals aged 6 months and older.[…]”
It took me a lot of reading to get a feeling for when they’re talking about one, the other, or both. You have to check every statement because it’s intentionally obscured and interspersed.
You can also find this abhorrent, demonic diagram. How much we knew by the time these products were brought to market, again and again.
There is even the clinical trial C4591017 comparing three “US lots” with one “EU lot”. The results are posted and it’s peculiar reading.
The EU lot compared to US Lot 3: 6098.6 (5474.7 to 6793.7) vs 6774.8 (6264.9 to 7326.1). The study only compares it to the pooled US lots (naturally). They also found the 20ug group to have higher titres 1 month post dose 2 than the 30ug group. You read that correctly.
Without going into it too much further, there’s also lots of data that was supposed to be calculated but was “abandoned at the sponsor’s discretion”. We can be grateful their first adapted booster design BNT162b2.B.1.351 was so terrifically shite it didn’t make it to market and it took them til Omicron to bust out the sequel (which ended up being half wild type anyway), at which point the disease was really not that scary any more and vaccine uptake had dwindled. Just one example:
That 2P-S sure is immunogenic, eh? Pretty hard to beat.
Correspondingly there is mention in the BioNTech SEC filings, for example this press release on a preprint about the bivalent variant contains the following sentence:
“The Pfizer-BioNTech COVID-19 Vaccines (COMIRNATY®) are based on BioNTech’s proprietary mRNA technology and were developed by both BioNTech and Pfizer. BioNTech is the Marketing Authorization Holder for BNT162b2 Wild Type and BNT162b2 Bivalent (Original/Omicron BA.4/BA.5) in the United States, the European Union, the United Kingdom, Canada and other countries, and the holder of emergency use authorizations or equivalents in the United States (jointly with Pfizer) and other countries.”
United States being mentioned twice highlights the difference between the products and their ownership. While BioNTech still get the money from the Pfizer-BioNTech Covid-19 Vaccine sales due to the profit sharing, the sponsor of the EUA products seems to be Pfizer. This is just my relatively lay understanding of the matter, and I realize it’s a slight contradiction to Robert Kogon’s work* concerning ownership of the vaccine. The Department of Defense contracts made public in Brook Jackson’s lawsuit show no mention of BioNTech, unless redacted. I discuss my theories about this and Moderna’s role in this twitter thread. No mention (that I know of) of “Pfizer-BioNTech Covid-19 Vaccine” in the BioNTech filings would make sense if BioNTech “have nothing to do” with the legally distinct EUA product. A closer look at BNTX filings might bring clarity but I’m already way over budget and past the schedule here.
*As he astutely notes, the EUA labelling retains the “Manufactured for: BioNTech” line, which is pretty conclusive dispositive evidence of my theory.
Documentation and production sites
This FDA page lists the authorization documentation (notice how they seem to be using Comirnaty and Pfizer-BioNTech interchangeably, but actually don’t). Unless I’ve missed it, none of these letters specify the authorized facilities except for Puurs, Kalamazoo and Andover in the August 23 2021 Comirnaty BLA, and Puurs, Kalamazoo and Hospira in the July 8 2022 Supplemental BLA. The first EUA references a total of six manufacturing “nodes”, and contains a section which essentially says “there are sites, we’ve looked at them and they’re fine”, but there is no mention of which sites those actually are. Only the labelling on DailyMed lists facilities.
These labels can be found on the DailyMed NIH website. Each listing includes the sites authorized to analyse, manufacture, package and label that product. Surprisingly, the authorized sites differ between versions. I’ve scraped the different labels and their authorized production sites into an excel table, along with all other sites I could find mentioned in the different approval letters, EMA news updates and FDA documentation.
The discrepancy in authorized sites in the FDA Comirnaty BLA and the sites listed in the corresponding DailyMed entry is obvious. I have not yet found FDA documentation naming the EUA authorized sites, I only have those from the labelling information.

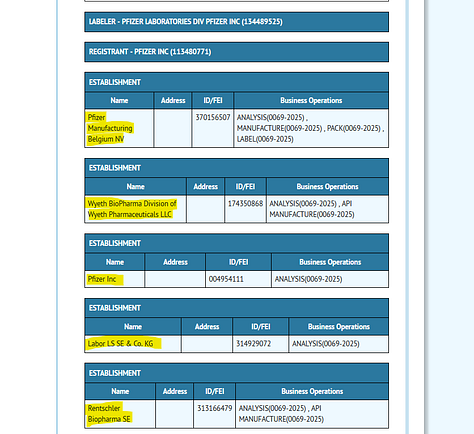

It’s all quite opaque.
Other countries
Canada and Australia are still using EUA labelled vaccine. UK seems to have used it (another search term is Regulation 174), and since switched to Comirnaty, not entirely sure though.
NZ seems to have been Comirnaty throughout, and its authorization lists three additional sites referenced in neither FDA nor EMA documentation. While Polymun is known from the clinical trials, its only authorized as a production site by New Zealand. The other two sites are also unique as far as I can tell, and the NZ sites only include Marburg, not Mainz, the main BNTX facility.
Are Comirnaty and Pfizer-BioNTech Covid-19 Vaccine just legally distinct, and do they only differ in certain inactive ingredients? Or does the inexplicably cost-inefficient partial overlap of production facilities and the peculiar clinical trial C4591017 hint at something more?
This is a work in progress simply due to the immense scope, and I will update the article with information as I find it.
Since publishing this article, Geoffrey Norman Pain on twitter has unearthed a Reuters photo from a vaccination center in Germany from January 2021 showing an EUA-labelled vial. This certainly disproves that Germany had Comirnaty labels throughout. When did we “switch”? How many EUA labelled vials were supplied? This supports his other finding, namely that the vials sold to Australia by Poland August 2021 were also “internationally” (EUA) labelled.
Update 23.4.2023: On 28.12.2022, South Africa approved “Cominarty”, API “COVID-19 mRNA VACCINE (embedded in lipid nanoparticles)”, supplied by Pfizer Laboratories (Pty) Ltd, registration date 25.1.2022, registration number 56/30.2/0002. The entries in the SAHPRA Professional Information/Patient Information Leaflet (PI/PIL) and Health Products databases where this information is from are not hard-linkable, so you’ll have to search for “cominarty”. The latter website has been changed, yet “SAHPRA” returns few-to-zero hits in archive site searches. The approval or EUA letter for the vaccine in SA has not yet turned up in my searches. The product was “approved for emergency use” (authorization and approval are distinct usually, unsure of SA pharmaceutical-regulatory terminology) on 17.3.2021, and the first batch arrived in the country on 3.5.2021. The Wikipedia article is a far greater help than the SAHPRA online offerings. So far it seems as if SA has had EUA-labelled Pfizer-BioNTech throughout, taking into consideration anecdotal reports of EUA-typical vaccination records.
24.04.2023: Just realized “tozinameran”, which is listed in SAHPRA consolidated medicine schedule also returns two hits on the astoundingly information-barren and user-unfriendly Health Products database: 1) Pfizer Laboratories (Pty) Ltd COMIRNATY Ready to Use Adult Vaccine 56/30.2/1179561179 2022/11/15 Registrered and 2) Pfizer Laboratories (Pty) Ltd COMIRNATY Dilute to Use Paediatric Vaccine 57/30.2/0022570022 2022/11/15 Registrered.
I’m quite curious if we’ll ever see a vial labelled “Cominarty”, but I wouldn’t hold my breath. I’ve found some other examples of “Cominarty” being used, ranging from eyebrow-raising to “probably just a mistake”: an official Brasilian document, a page by the US Coast Guard, an official document from Germany’s Kassenärztlicher Vereinigung, the central body of doctors licensed to bill insured patients, a few mentions in German press, and by public broadcaster mdr. But no mention of anybody having a problem with (one of) South Africa’s versions being spelt differently.
06.05.2023: Looking into the bizarre “Domirnaty” sculpture made from empty vax vials by a German GP, one of the vials on the bottom of the sculpture is clearly EUA-labelled. The 220 vials were collected between January and August 2021 according to this article and the practice’s website. The article is from mid September, the website seems to say the piece was finished beginning of August 2021. As outlined in the twitter thread, there are notable differences in vial size between top and bottom of the sculpture. If anybody knows what the blue-bordered label on a couple of vials means, feel free to let me know.









“Curious Kittens”, Nepelactalone Newsletter
https://anthraxvaccine.blogspot.com/2021/08/are-fda-and-pfizer-biontech-scamming-us.html
https://childrenshealthdefense.org/defender/no-one-can-force-you-pfizers-comirnaty-vaccine/





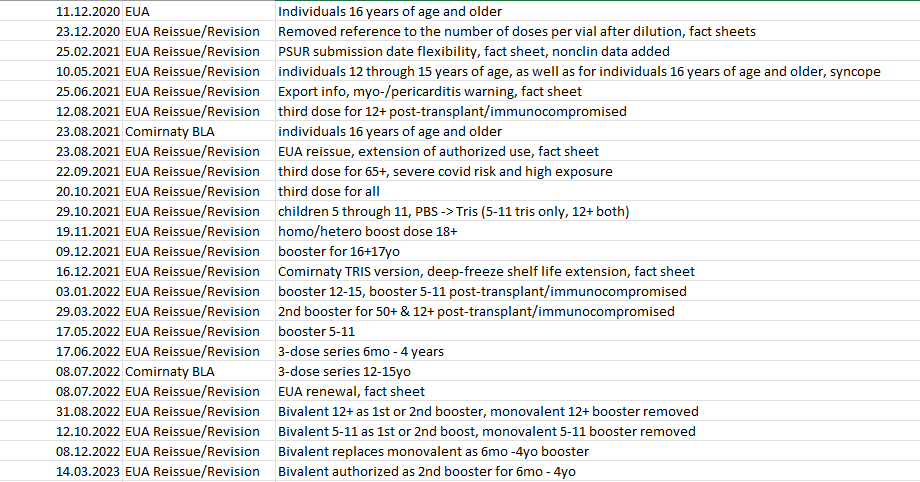
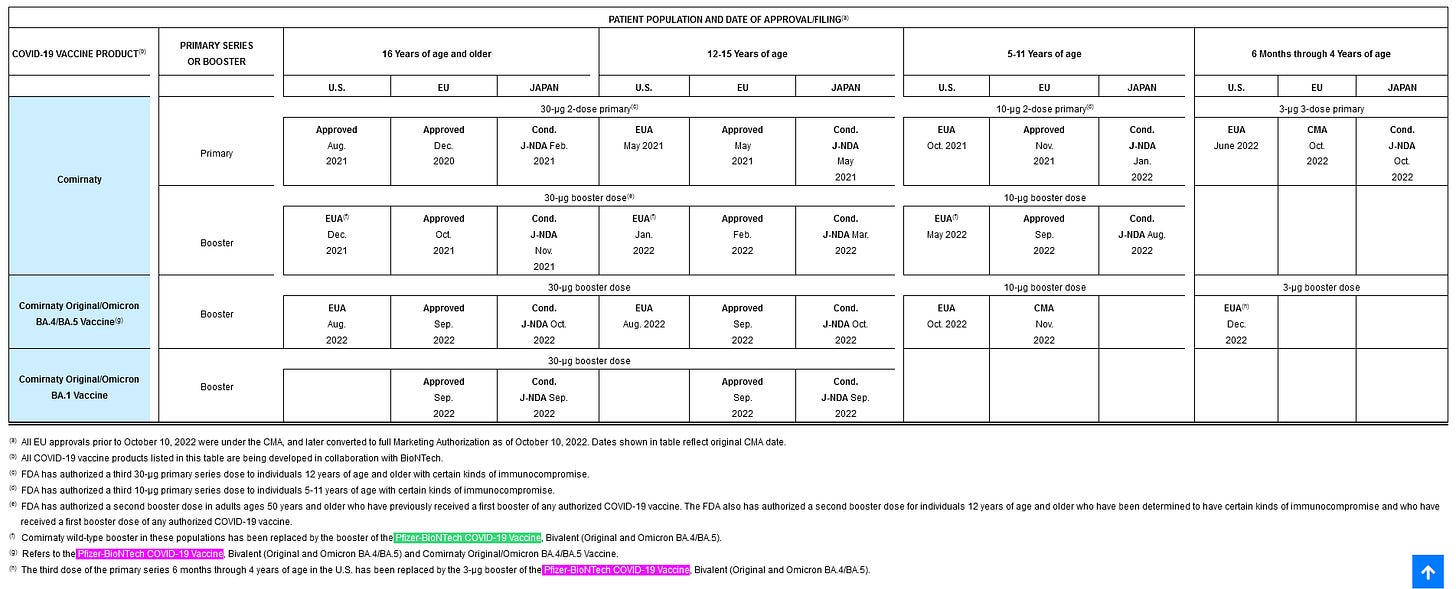






Very interesting. Thank you.
Very interesting thanks, not just because I see myself mentioned along with a link to my Twitter page.
https://geoffpain.substack.com/